Coagulation
Coagulation is the step that starts
off the treatment process. In
coagulation, the dirty water is placed in a single container and has a
coagulant (chemical) mixed in with it all.
This chemical can be multiple things, but is typically called alum. Aluminum sulfate, commonly known as alum, is
a chemical that removes negative charges from whatever compounds it is mixed in
with.
After the alum is added, a fan will
evenly disperse the chemical in with the dirty water. With the addition of positive charges and
negative charges taken away, the loose particles in the water are more likely
to stick together in large clumps. The
faster the mixing the better as speed of mixing won’t influence how the clumps
stick together, but insufficient speed will definitely influence size of
clumps, later influencing the effectiveness of later processes. Coagulation tends to only take about 1 to 3
minutes.
Flocculation
Flocculation is the next step in
water treatment and receives the batch of water that just underwent
coagulation. Similar to coagulation,
flocculation is a mixing stage and is purely dedicated to mixing.
Flocculation typically involves
multiple mini stages of varying levels of intensity of mixing. The coagulated water has very tiny clumps of
dirt particles that are stuck together but nearly invisible to the human
eye. The assorted levels of mixing in
flocculation allow the tiny particles in the water to slowly clump together
into larger and larger groups with each successive pass. This process can take anywhere from 15
minutes to a full hour depending on the design plans and end goals.
Once the clumps are of ideal weight
and size the next phase of treatment can begin, filtration.
Filtration
Cross
flow membrane filtration removes both salts and dissolved organic matter, using
a permeable membrane that only invades the contaminants. The remaining water flows
along across the membrane and out of the system and the contaminants are
removed as it flows along the other side of the membrane.
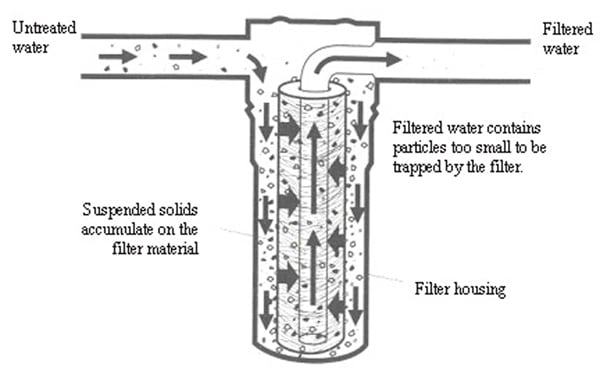
Usually at the start of the process, filtration through screens occurs. The shape of
the screens depends on the type and size of particles that have to be removed.
Sand filtration is a frequently used, very vigorous
technique to remove suspended solids from water. The filter medium consists of
multiple layers of sand. When
water flows through the filter, the suspended solids precipitate in the sand
layers as residue and the water, which is reduced in suspended solids, flows
out of the filter. When the filters are loaded with particles the
flow-direction is switched, in order to regenerate it. Smaller suspended solids
have the ability to pass through a sand filter, so that secondary filtration is
often necessary.
Microfiltration, ultrafiltration,
and nanofiltration are all membrane separation techniques in which very fine
particles or other suspended matters are separated from a liquid. The
difference between the three is the size of the particles that can be
separated. Microfiltration removes the largest particles, such as suspended
solids and bacteria. Ultrafiltration removes those slightly smaller, such as
salts and proteins. Nanofiltration removes the smallest particles, such as
pesticides, herbicides, and viruses. Reversed osmosis has the same function,
although it is capable of removing metal ions and aqueous salts.
http://www.filtersfast.com/articles/ArticleImages/sediment-filtration.jpg
http://www.lenntech.com/water-purification-steps-faq.htm
Disinfection
The main goal of water treatment is
to ensure that the water is safe to drink and does not contain any
disease-causing microorganisms after purification.
In order to meet this goal, a water
technique called disinfection is used to purify the water. Disinfection is the
chemical process used to remove, deactivate, or kill pathogenic microorganisms
and/or disease causing bacteria in the water. Disinfectants must also remain
active to prevent recontamination after the water has been disinfected.
Some chemical disinfectants often
used to disinfect water are chlorine, iodine, bromine, sodium hydroxide and
lime (bases), and ozone. These chemicals are put in the water to react with and
kill pathogenic microorganisms present in unpurified water. Other non-chemical
methods of disinfection include the use of ultraviolet lights, which kill
dangerous microorganisms and bacteria in order to cleanse the water. Heat is
another form of non-chemical disinfection which essentially destroys all
microorganisms and disease-causing bacteria in the water. All methods of
disinfection can be used to ensure safe drinking water and safe use of water
for people.
http://www.lenntech.com/process
es/disinfection/what-is-water-disinfection.htm#ixzz2ga8bHmak


What is the difference between coagulation and flocculation?
ReplyDeletein coagulation, impurities are removed from the water using a coagulant. in flocculation, the tiny particles that are left behind from coagulation then clump together before the water moves on to be filtered
ReplyDeleteIn different regions of the country, are water filtration techniques in any way, different?
ReplyDeleteCan any of these techniques be used long-term or are they just temporary? Do you think any of these could advance into something more permanent if not?
ReplyDeleteI believe charcoal is a common filtration media. Why is this?
ReplyDeleteWhat short term or long term goods or services can provide these purification techniques?
ReplyDeleteDoes coagulation rid the water of disease?
ReplyDelete